Oakgrove Insights
There is always a better way
Delivering actionable insights across rare disorders. Biopharma innovators need accurate, comprehensive and time series insight for rare disease disorders. Today there exists a wide information gap between rare disease data and the required analytics demanded by pharma discovery teams, development leaders, medical teams and commercial engagement professionals.
About
Oakgrove Insights is dedicated to advancing the application of real world data.
Critical data leading to actionable insights is required throughout the entire drug development and commercialization process.
- Clinical teams start the trial process with the submission of the Investigational New Drug Application (IND).
- Requirements – detailed patient journey to identify the largest area of unmet need and best align trial design with need, selecting critical and measurable endpoints, etc.
- Medical teams seek to engage critical thought leaders with the latest insights and treatment revelations.
- Requirements – collaborative discussions on patient unmet need, gaps in current understanding, and the need to reach underserved patient population. How to share these important insights with key opinion leaders.
- Commercial teams prepare for launch.
- Requirements – define the specific patient cohort, the HCPs engaging this cohort, key decision points in the patient journey, benefits and limitations of current therapies and how best to position the novel agent vs current therapies.
The Oakgrove Insights Process
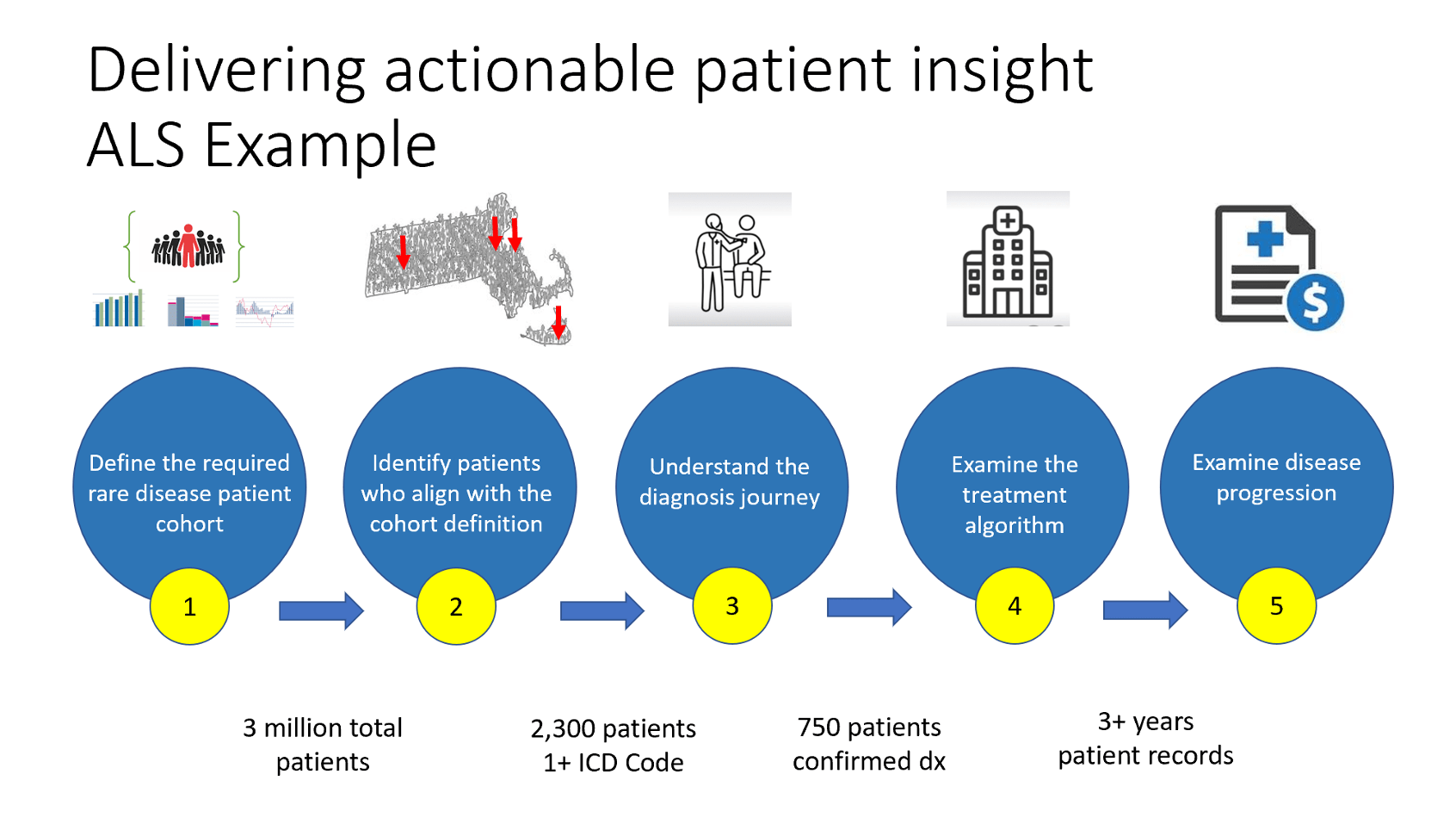
OAKGROVE RARE is an online dataset that is purposedly build to address the challenges of rare disease. Efforts start with defining the required patient cohort, identifying the patients who align with the requirements, understanding the patient’s journey to diagnosis, creating algorithms based on the diagnosis journey. Once defined OAKGROVE RARE provides teams with exceptional insight on disease progression, therapy selection and how to apply the learnings to a claims data sets.
Example Case Study – OAKGROVE RARE for amyotrophic lateral sclerosis (ALS) diagnosed patients.
Define the rare disease Patient Diagnosis Journey
- Examine ALS diagnosis patient journey
- Review patients that received a single diagnosis code but never diagnosed
- What tests were ordered, which procedures were conducted?
- What diagnosis was provided? Which diagnoses were ruled out?
- Confirmed diagnosed of ALS patients
- Which tests were ordered, which procedures conducted, what were the results?
- Which HCP (specialty) made the diagnosis?
- Which disorders were ruled out?
- Which results were most critical to making the diagnosis?
- What symptoms were reported (based upon frequency)?
- Examine the pre-diagnosis journey and define time to diagnosis?
- Review patients that received a single diagnosis code but never diagnosed
Define the Treatment Algorithm for Rare Disease
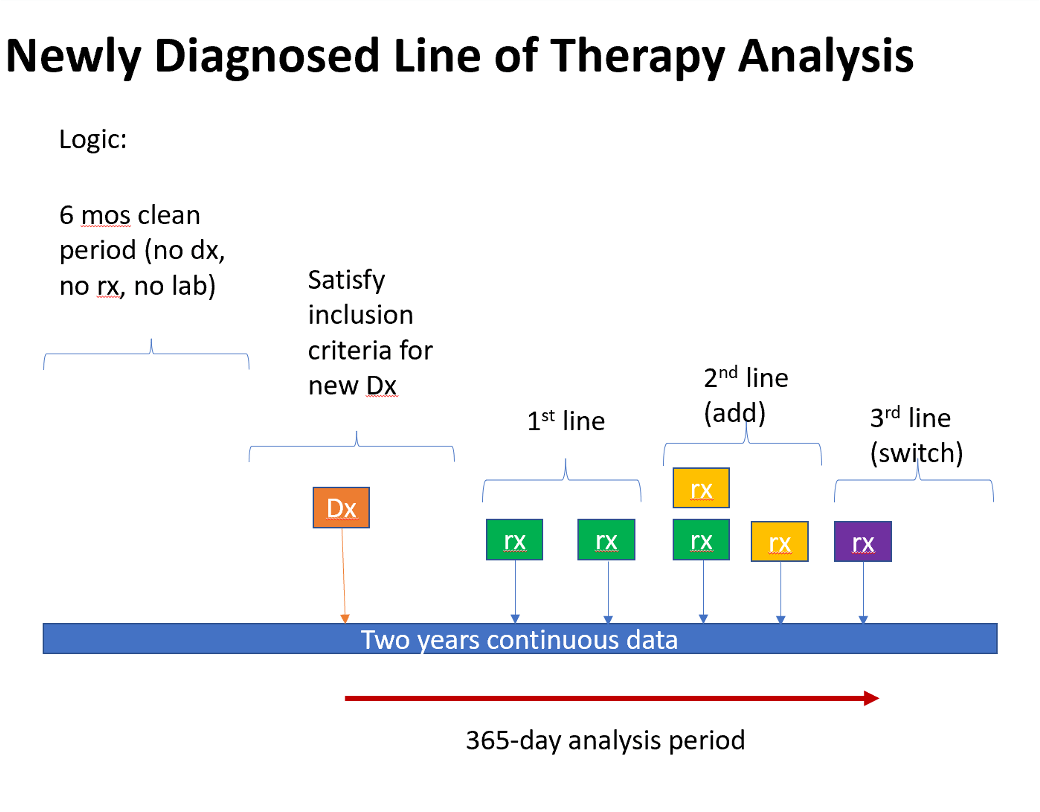
- Examine newly diagnosed patients on a go forward analysis
- What was the initial therapy started after diagnosis
- How long did patients stay on the initial therapy?
- What was second line therapy?
- Examination of adds, switches and the reason provided for change.
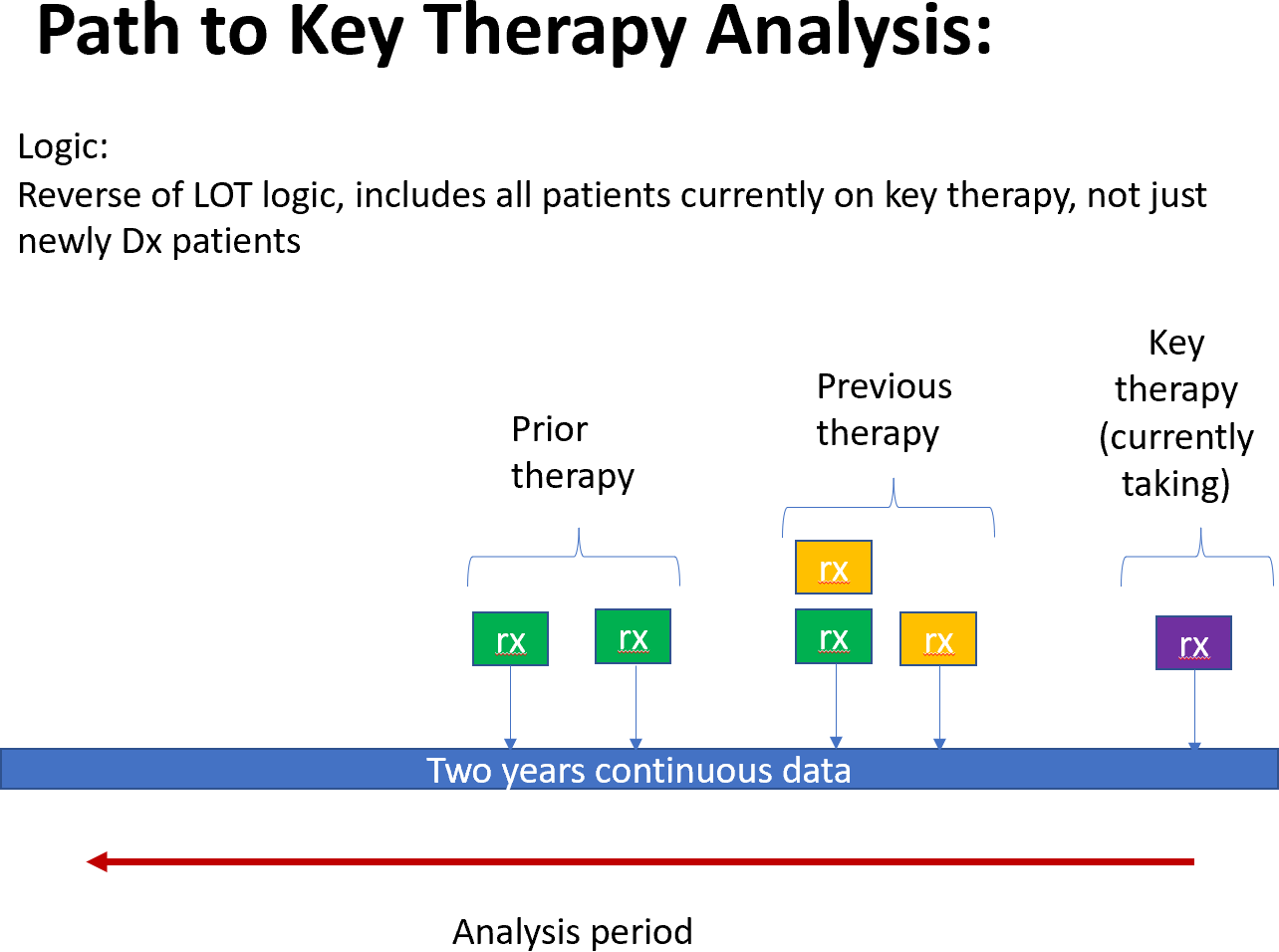
- Examine the pathway to key therapies – a retrospective analysis
- How did rare disorder patients get to a specific therapy
- Examine critical steps to each therapy and the decision points along the journey.
Get in Touch
Email Us
info@oakgroveinsights.com
Phone
+1 (617) 780-7615
Office
240 Main Street
Suite 301A
Bridgewater, MA 02324